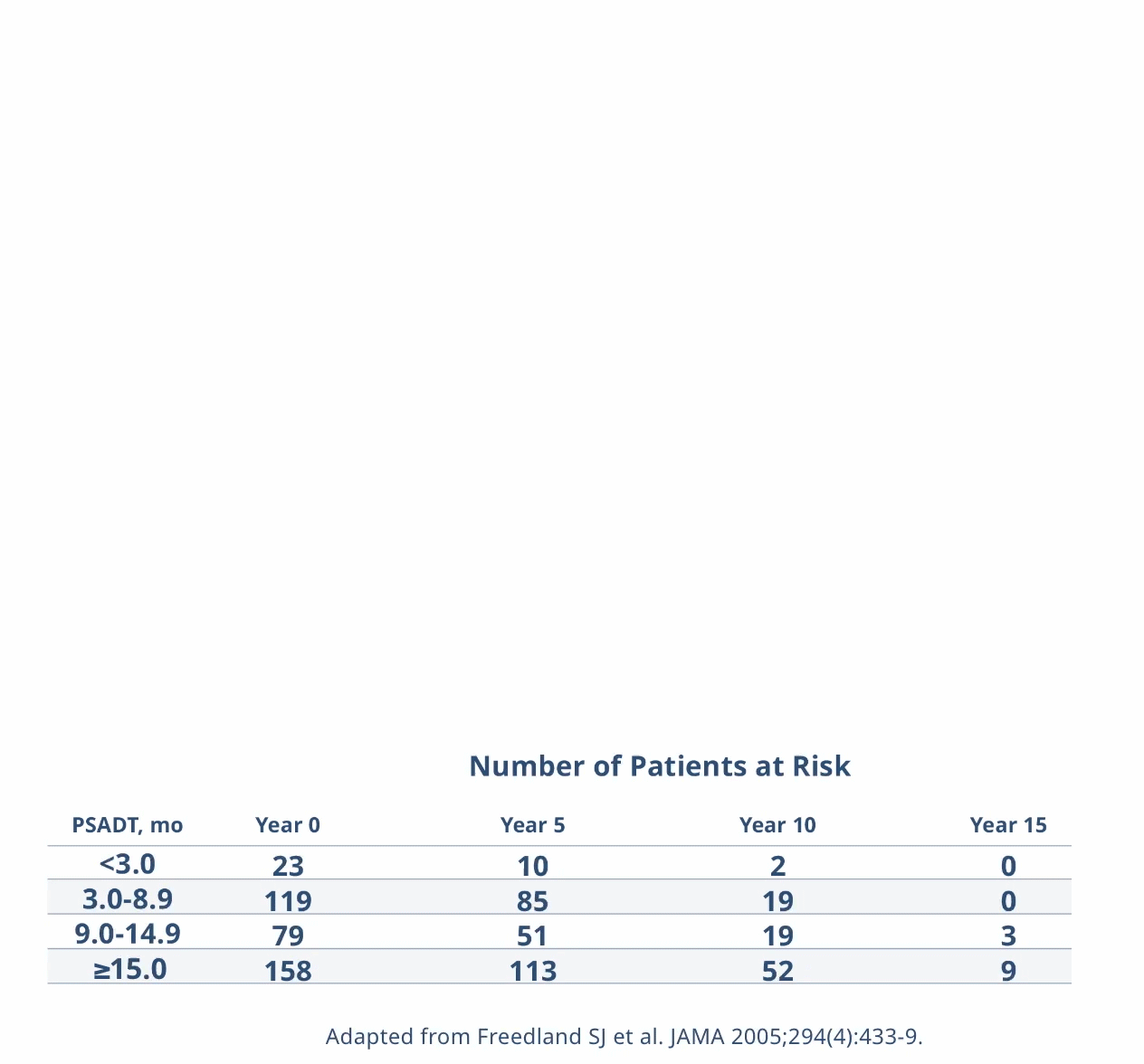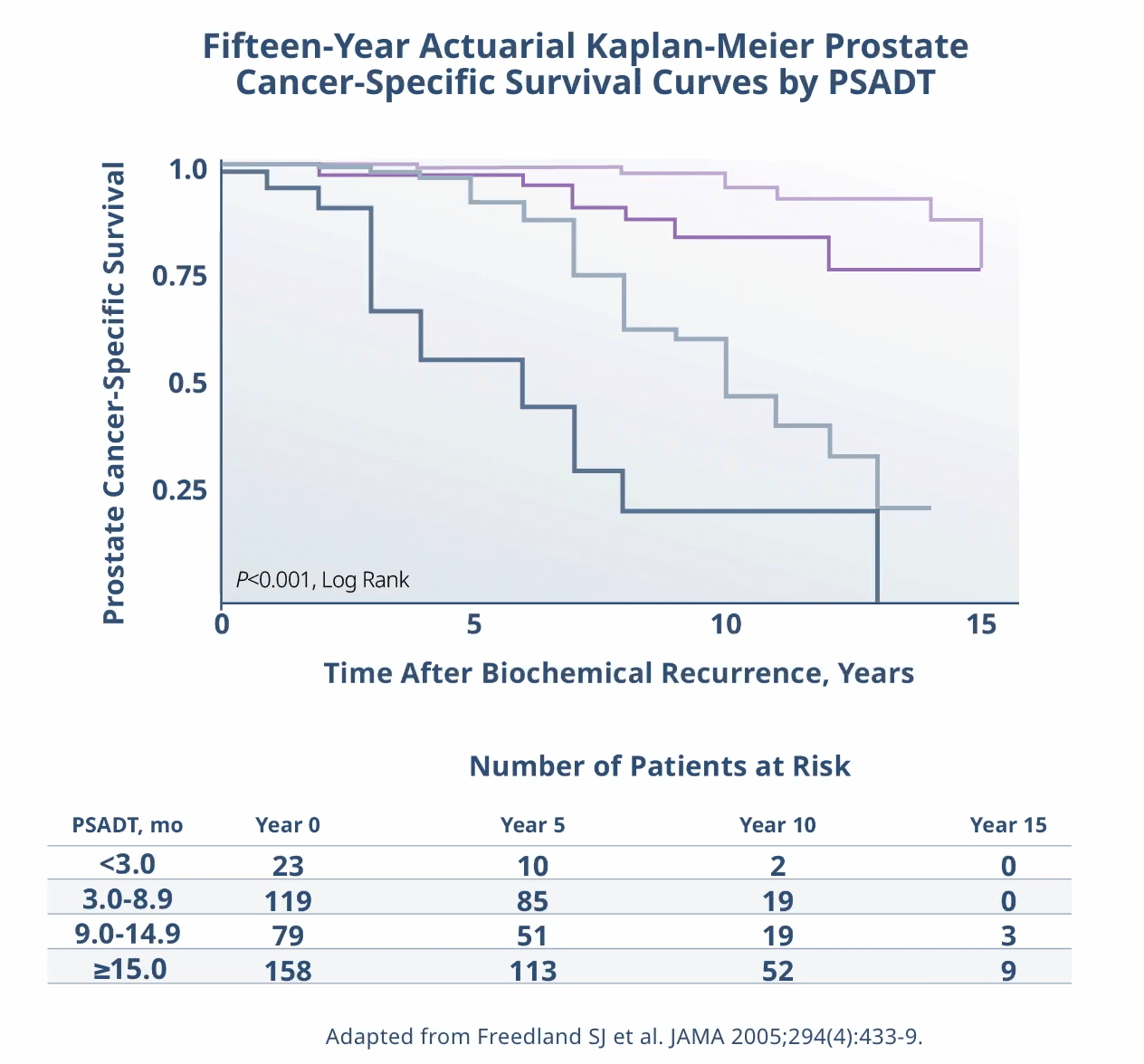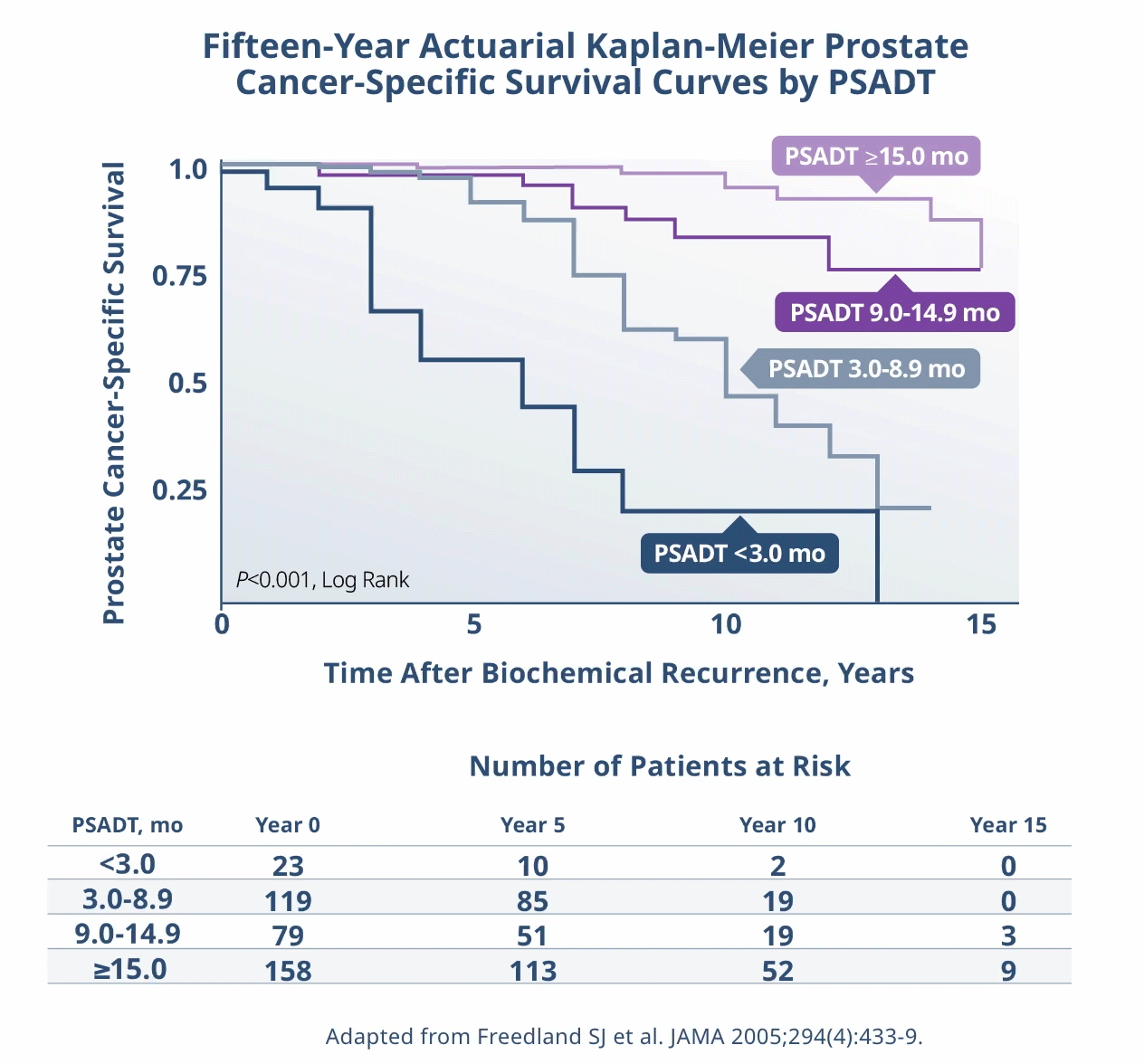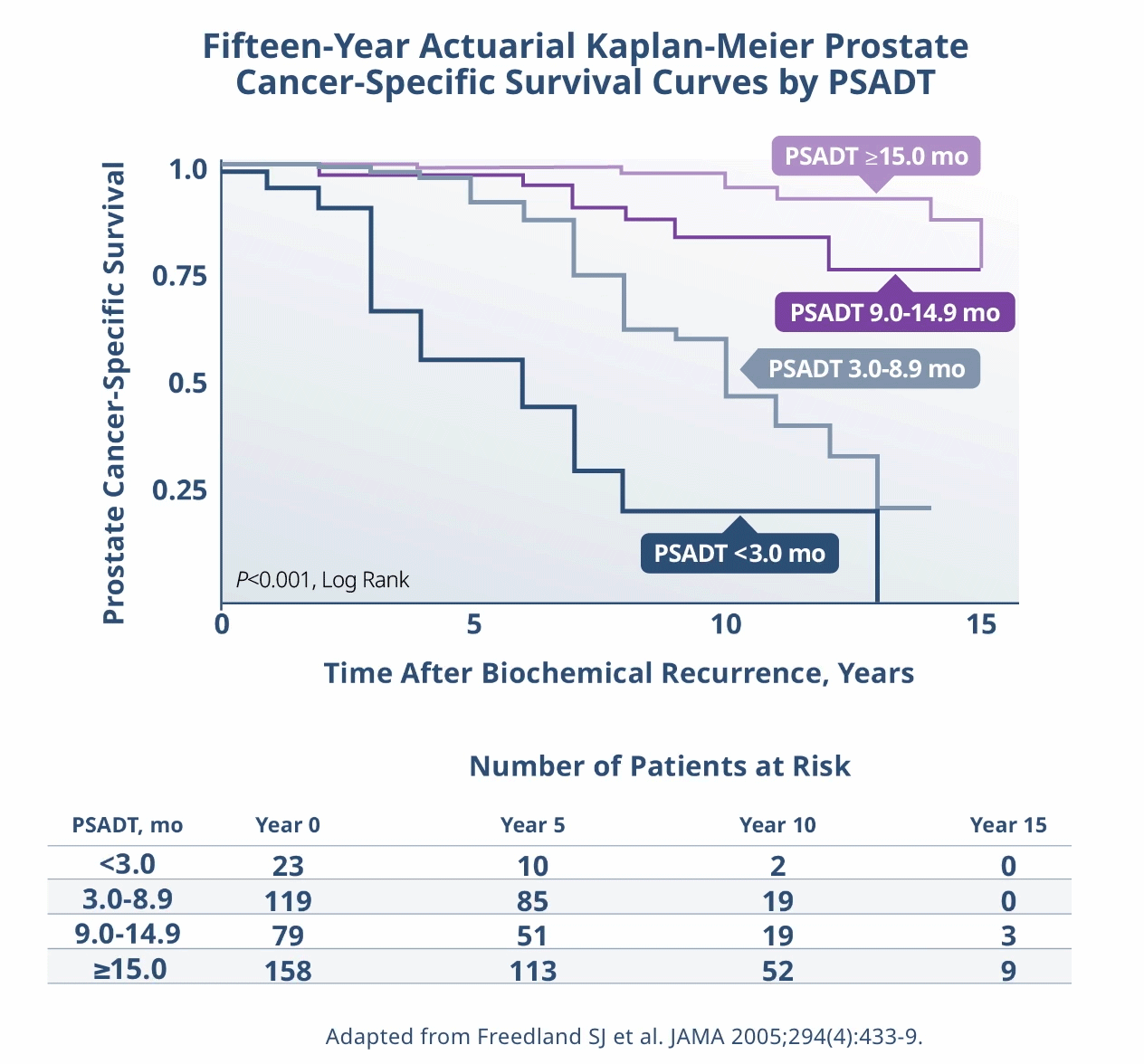
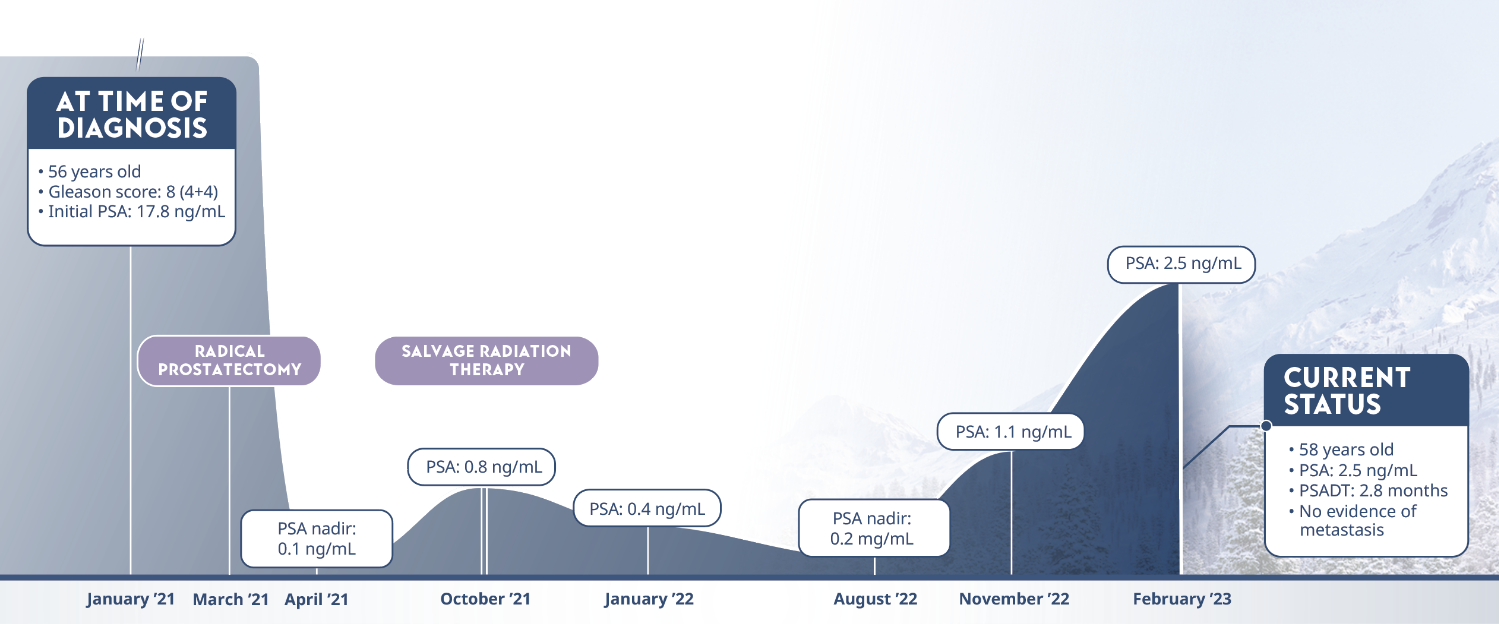
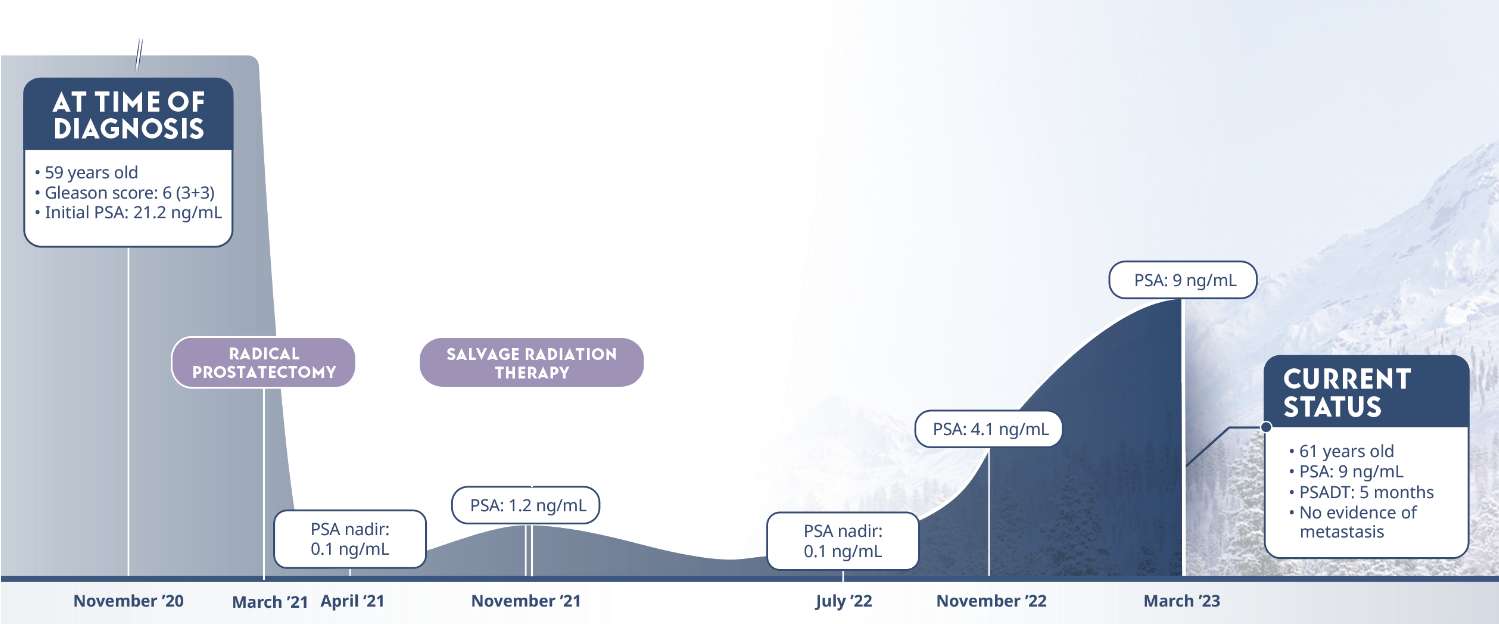
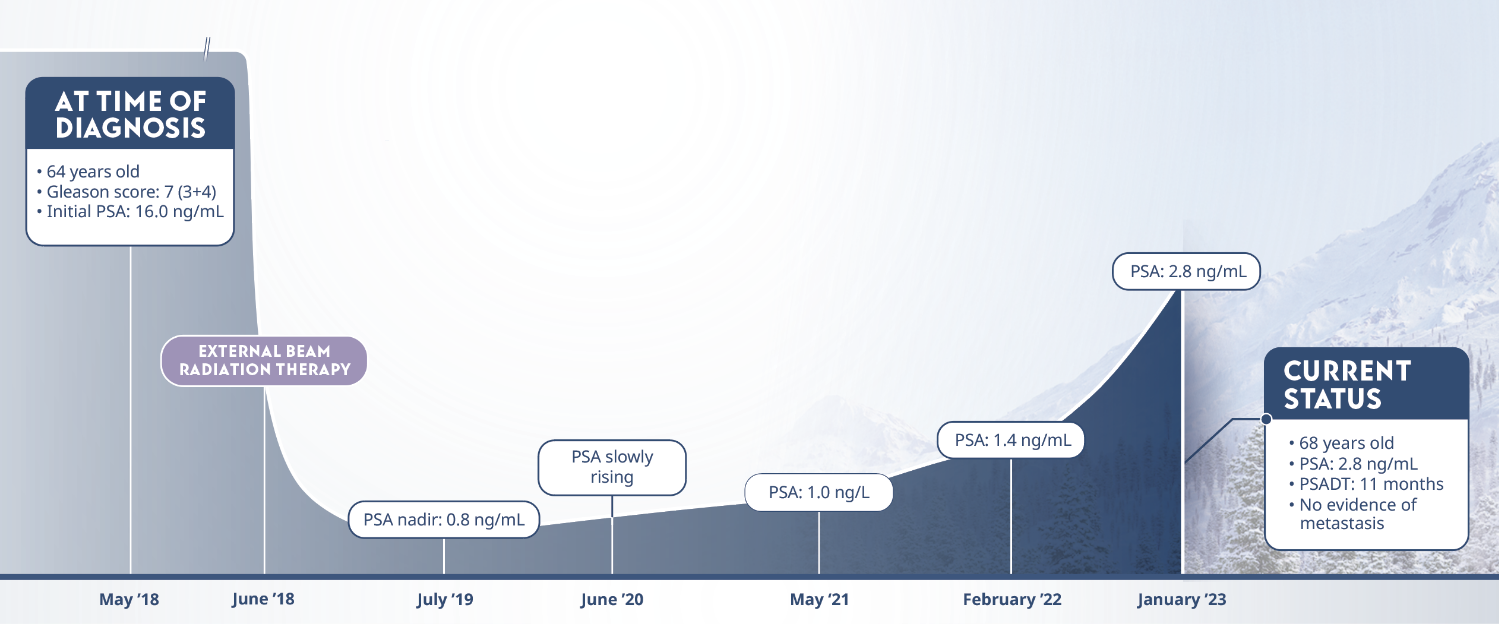
Limitations: This study was subject to the limitations of retrospective cohort studies, which include data quality factors (e.g., missing data) and loss of patients to follow-up. Because this study was not randomized, its results are subject to potential confounding by unmeasured variables and sampling biases. The authors identify other limitations of the study, including (1) small subgroup size and large confidence intervals; (2) a predominantly white population limiting generalizability of results to a more racially diverse population; and (3) calculation of PSADT based on the assumption that PSA levels increase exponentially (at least during the first 2 years after recurrence), although it is plausible that, in the long term, PSADT values may not be stable. They note that external validation from other, similar studies, or from prospective clinical trials is necessary.
†Prostate cancer death was defined in this study as death occurring in a patient who had metastases that progressed following hormonal therapy.5